John Lopresti, former researcher from Agriculture Victoria, discusses research on table grapes cool storage performance, part of the Serviced Supply Chain Project
Video transcript: Table grapes cool storage performance 2021
Of major interest to table grape growers and exporters, is the difference in grape cultivars in terms of post-harvest storage potential and performance and quality loss. And from previous work internationally and locally, we know that some grape cultivars more prone to developing rots and other quality loss, such as rachis browning, or rachis drying and discoloration.
To summarize that workers shown there is a moderate to high effect of cultivar on subsequent storage quality and quality during export, and this has to be taken into consideration when growers and exporters look at what markets to send their fruit to and how to treat their fruit, prior to marketing. And this information just provides another means for growers to assess the risk of quality loss in their various cultivars as prior to storage and marketing.
Factors influencing table grape cultivar storage potential
- Table grape cultivars are susceptible to various fungal rots
- Susceptibility to postharvest rot development is cultivar-dependent
- SO2 treatment appears to have minimal impact on postharvest rachis browning severity
- Within a cultivar,
- high variability in susceptibility to fungal rot development is likely within and between cartons
- vineyard factors including bunch disease latent infection and inoculum load at harvest greatly influence postharvest rot development
- Appropriate use of SO2 treatment of all cultivars substantially reduces the potential for rot development during extended cool storage or along export cool chains.
On this page:
- Background
- Table grape quality loss during storage or export
- SO2 treatment of ‘Crimson Seedless’ and ‘Sweet Sapphire’ for export
- Berry shatter
- Rachis browning
- Bunch rot incidence
- ‘Thompson Seedless’
- Conclusions
Background
The table grape industry is highly dependent on sulphur dioxide (SO2) generator pads to prevent or reduce quality loss within domestic and export supply chains. SO2 pads are generally effective in preventing rot development; however, their use rarely takes into account vineyard agronomic and disease control practices, the degree of latent infection in harvested fruit, differences in susceptibility of grape cultivars, potential for berry and rachis bleaching, and supply chain conditions.
Table grape quality loss during storage or export
Due to a relatively small domestic market and distant export markets, the period between harvest and marketing can be up to eight weeks during which time table grapes are cool stored and/ or exported at temperatures below 3°C. Although table grapes are a non-climacteric fruit with a relatively low rate of physiological activity, eating and visual quality can be reduced significantly by poor temperature management and excessive water loss. Fungal rot development, rachis browning, berry shrivel, and berry shatter (i.e., berry detachment from rachides) are usually the main contributors to postharvest quality loss among table grapes. In many cases rachis browning is the first symptom that limits bunch marketability due to high susceptibility of rachides to water loss, and high respiration rate of the rachides relative to the waxy grape berries. Obviously, this browning does not impact on berry eating quality, but from an importer and consumer perspective, bright green rachides are an important indicator of bunch freshness and remaining shelf life.
The risk of fungal rot development during cool storage and marketing increases with high disease inoculum in the vineyard, higher storage temperatures and longer storage duration. There are five main postharvest rots that impact on grape quality: Botrytis cinerea (Grey mould), Alternaria alternata (Alternaria rot), Aspergillus sp. (Aspergillus rot), Penicillium expansum (Blue mould), and Rhizopus sp. (Rhizopus rot). The above fungi are saprophytic as they tend to remain on the berry surface with rot symptoms expressed once grape berries are either damaged or develop fine cracks as they soften, or at the attachment of berries to rachides once berries begin to loosen due to water loss and longer storage durations. Sulphur dioxide pads (i.e., sodium metabisulphite) are used as standard commercial practice during storage or export and are generally effective in minimising rot development, although their efficacy can depend on cultivar, inoculum levels in the vineyard and berry damage among bunches during export. Botrytis can also infiltrate fruit at flowering if timing of fungicide applications doesn’t protect flowers from infection and remain latent in berries until it is expressed during postharvest storage, particularly if temperature management is poor, or during distribution or marketing where higher storage and handling temperatures can accelerate rot development.
Table grape cultivar can also impact on storage potential, and specifically on rachis browning, shatter and rot development during storage (Lichter et al., 2008; Crisosto et al., 1994). Below we present results from a sea freight export simulation study that compared the impact of SO2 treatment and storage duration (i.e., export stage) on shatter, rachis browning and rot development in ‘Crimson Seedless’ and ‘Sweet Sapphire’ grapes. Results from these two cultivars were compared to experiments using ‘Thompson Seedless’, a white cultivar that is considered more susceptible to fungal rots during storage than red or blue cultivars.
SO2 treatment of ‘Crimson Seedless’ and ‘Sweet Sapphire’ for export
Sea freight export simulation, including importer storage, distribution and marketing stages was conducted for commercially pre-cooled and packed ‘Crimson Seedless’ (Fig. 1) and ‘Sweet Sapphire’ (Fig. 2) grapes. Fruit was harvested from the Sunraysia region in Victoria. Sea freight for four weeks was simulated at 0 °C, with an importer storage stage of two weeks at 2 °C, distribution stage of one week at 4 °C, and finally, a marketing period of three days at 18 °C. For each cultivar, export simulation was conducted on five cartons of grapes treated with an SO2 pad, that was removed prior to the marketing stage, and on five cartons without SO2 pads (i.e., untreated controls).

Figure 1. SO2-treated ‘Crimson Seedless’ grape quality after simulated sea freight, importer storage and distribution.

Figure 2. SO2-treated ‘Sweet Sapphire’ grape quality after simulated sea freight, importer storage and distribution.
Incidence of berry detachment from rachides (i.e., berry shatter) was determined for each bunch by holding bunches from the top of the main stem and gently shaking it for five seconds. Shatter incidence was then calculated as the proportion of loose berries by weight. A five-point rating scale was used to assign a rachis browning score to bunches where 1 = rachis completely green; 2 = only pedicels yellow/brown; 3 = partial browning of laterals and mostly brown pedicels; 4 = all pedicels and laterals brown; and 5 = all rachis completely brown. Incidence of individual berries with fungal rot symptoms within a bunch was determined destructively at all removals by detaching and weighing all berries with symptoms of Botrytis cinerea and other common rots, and then calculating rot incidence as a proportion of total bunch weight.
Berry shatter
Berry shatter was found to vary little among ‘Crimson Seedless’ and ‘Sweet Sapphire’ with an incidence of less than 10 % in both treated and control fruit along the simulated export chain including a marketing period at 18 °C (Fig. 3a and 3b). In both cultivars the difference in shatter incidence between treated and control fruit was minimal, indicating that berry loosening from pedicels during storage and marketing, due for example to water loss, is unlikely to reduce bunch quality in either cultivar. SO2 treatment significantly reduced shatter incidence in ‘Crimson Seedless’ after the distribution stage but not in Sweet Sapphire. No shatter assessments were conducted after the marketing stage of untreated bunches.
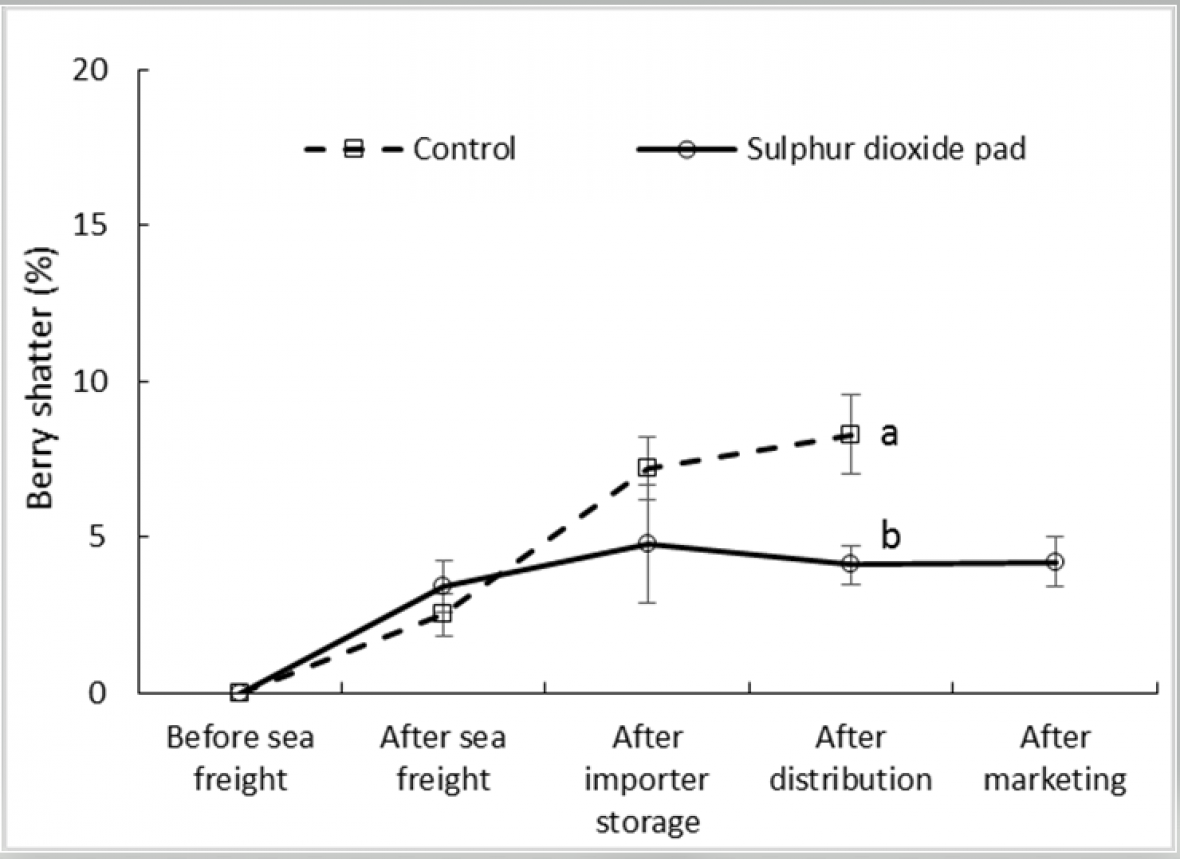
Figure 3a. Effect of SO2 treatment and export stage on incidence of berry shatter in ‘Crimson Seedless’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001.
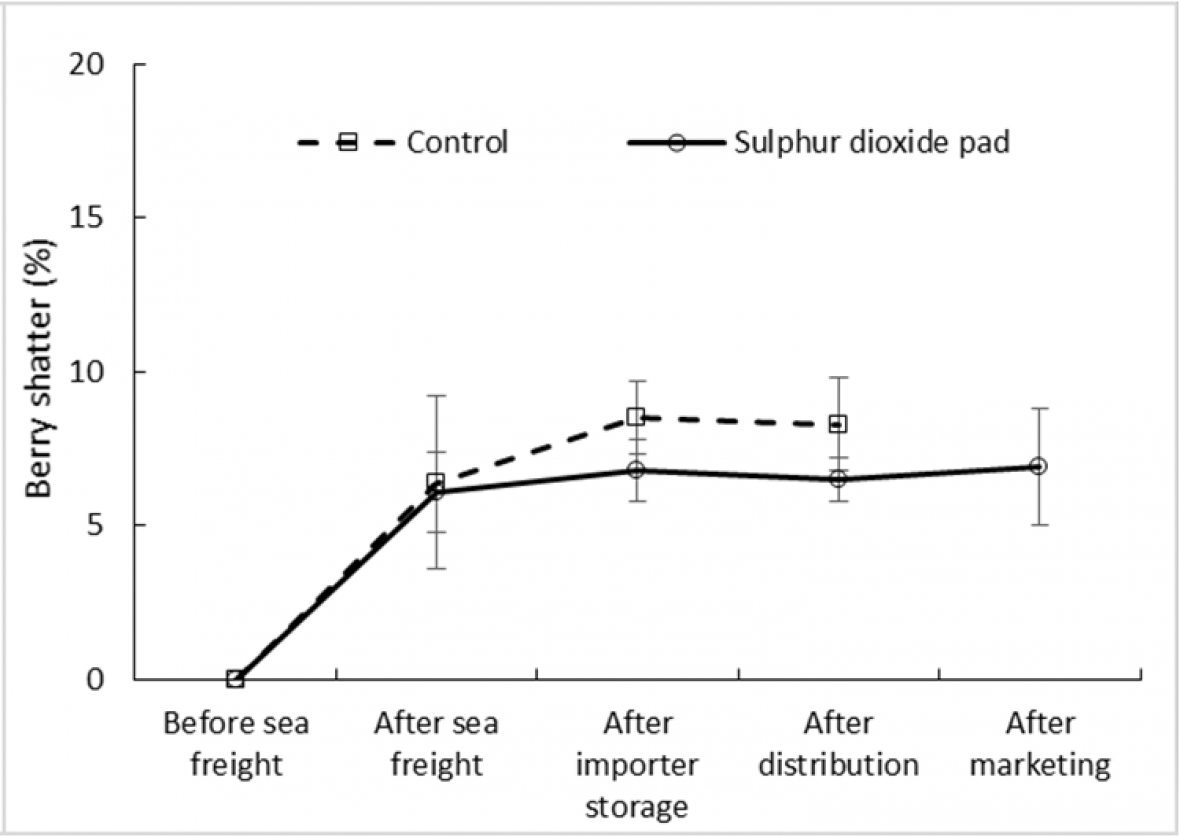
Figure 3b. Effect of SO2 treatment and export stage on incidence of berry shatter in ‘Sweet Sapphire’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001.
Rachis browning
A comparison of rachis browning severity in treated ‘Crimson Seedless’ and control bunches during simulated export and marketing shows that SO2 treatment made little difference to the rate of browning. Fruit from both treatments reached the limit of marketability during importer storage and distribution stages (Fig. 4a and 4b). Similarly, no significant difference was observed in browning severity within treated and control ‘Sweet Sapphire’ bunches along the simulated export chain, with fruit only reaching the limit of marketability after the marketing period at 18 °C. These results suggest that ‘Sweet Sapphire’ is very robust in terms of quality loss due to rachis browning and appears to be less prone to browning than ‘Crimson Seedless’.
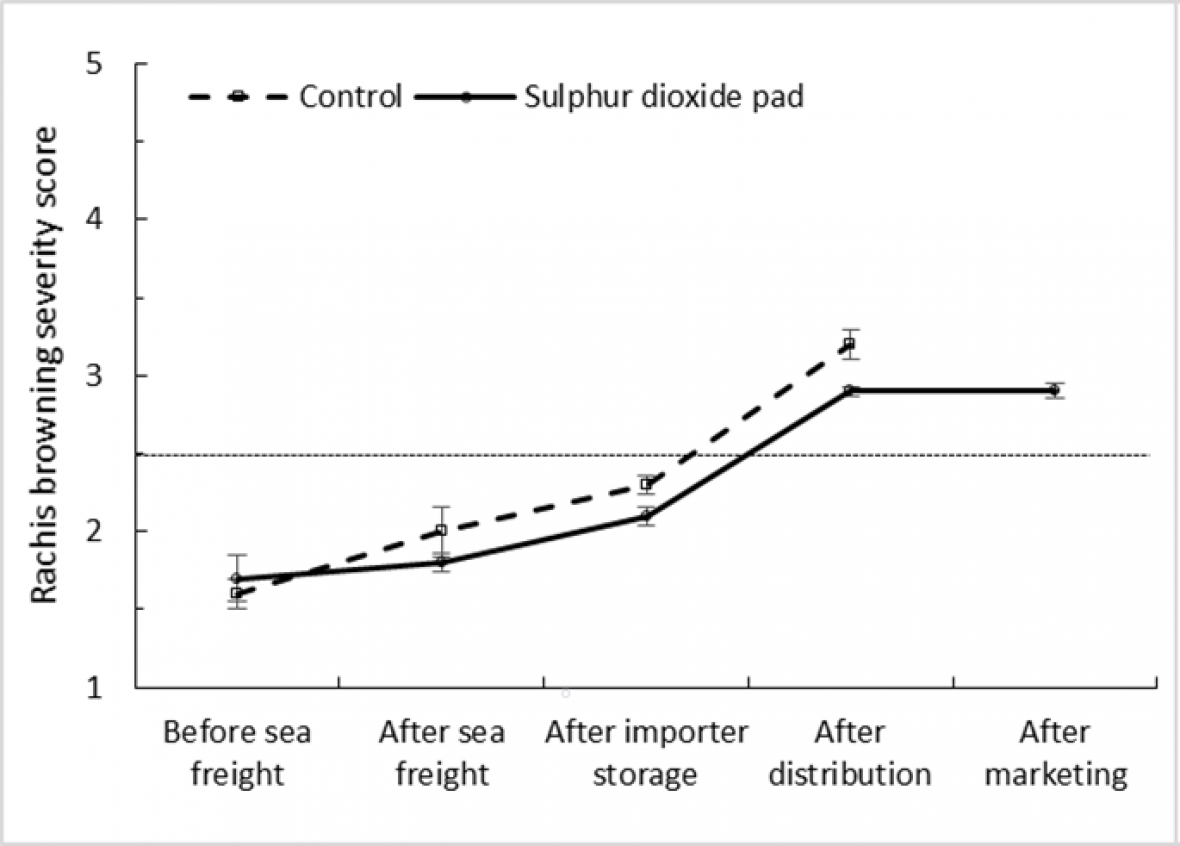
Figure 4a. Effect of SO2 treatment and export stage on rachis browning severity score in ‘Crimson Seedless’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001; dotted horizontal line represents bunch ‘limit of marketability’ based on browning severity.
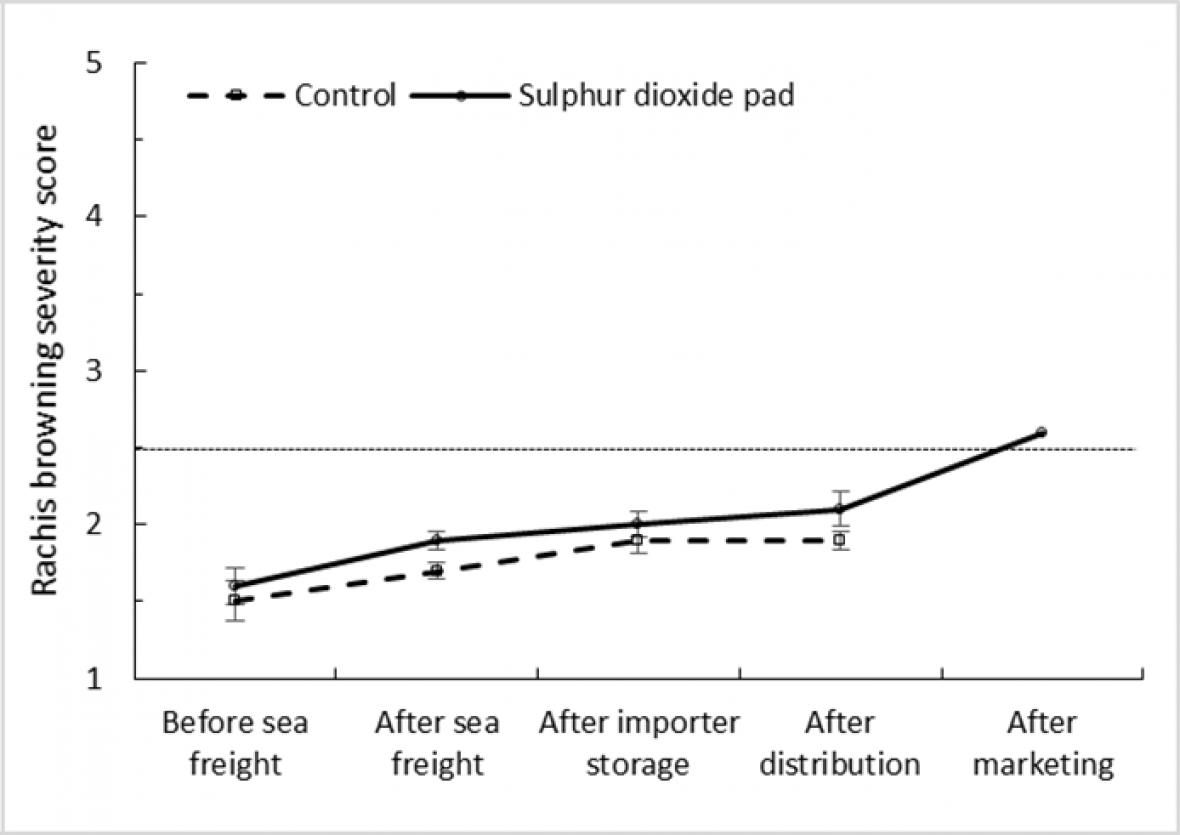
Figure 4b. Effect of SO2 treatment and export stage on rachis browning severity score in ‘Sweet Sapphire’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001; dotted horizontal line represents bunch ‘limit of marketability’ based on browning severity.
Bunch rot incidence
As expected, SO2 treatment reduced rot incidence in bunches compared to untreated fruit in both ‘Crimson Seedless’ and ‘Sweet Sapphire’ during export simulation. Differences between treatments only became apparent and significant after importer storage and after distribution in ‘Crimson Seedless’ and ‘Sweet Sapphire’, respectively (Fig. 5a and 5b). In both cultivars SO2 maintained bunch rot incidence below 2% until the end of the distribution stage, with rot incidence among untreated bunches significantly greater after this export stage. ‘Crimson Seedless’ rot incidence increased rapidly after the sea freight leg, whilst this rate of increase was lower in untreated ‘Sweet Sapphire’ fruit, with relatively wide error bars suggesting that for both untreated cultivars, rot development was highly variable in each replicate carton.
The majority of rot development in ‘Crimson Seedless’ was due to Botrytis cinerea whereas the observed rot in Sweet Sapphire was mostly due to other saprophytic fungi such as Penicillium expansum and Rhizopus sp. This result indicates that disease development within ‘Sweet Sapphire’, particularly during marketing, was mainly induced by berry damage and skin cracking. Vineyard management may also have been a factor by influencing initial disease levels on bunches at harvest.
The potential variability in rot susceptibility within cultivars is clearly demonstrated by incubation studies at harvest where berry samples from five cartons were incubated for six days at 18 °C (Table 1). In both cultivars used for this study, a high coefficient of variation was observed within cartons based on rot incidence in four bunch samples per carton, with moderate variation between cartons. Botrytis cinerea was the main rot observed. Rot variability within and between cartons was higher in Sweet Sapphire fruit indicating that in this cultivar, relatively minor differences in berry cracking and damage between bunches can allow entry of saprophytic fungi such as Alternaria rot and Blue mould. Under these circumstances, low latent Botrytis in berries does not preclude bunches from developing rot symptoms due to these other fungi.
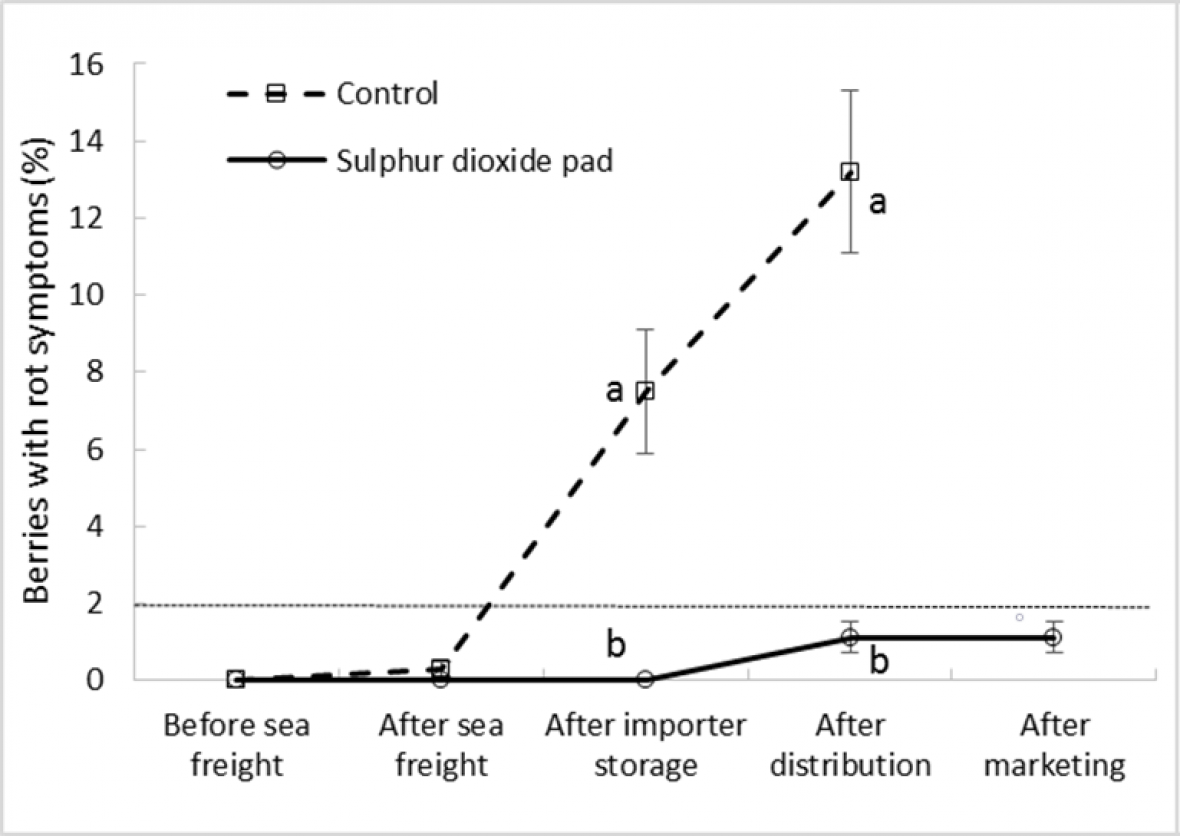
Figure 5a. Effect of SO2 treatment and export stage on bunch rot incidence in ‘Crimson Seedless’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001; dotted horizontal line represents bunch ‘limit of marketability’ at 2 % incidence (i.e., approximately 10 berries with rots per bunch).
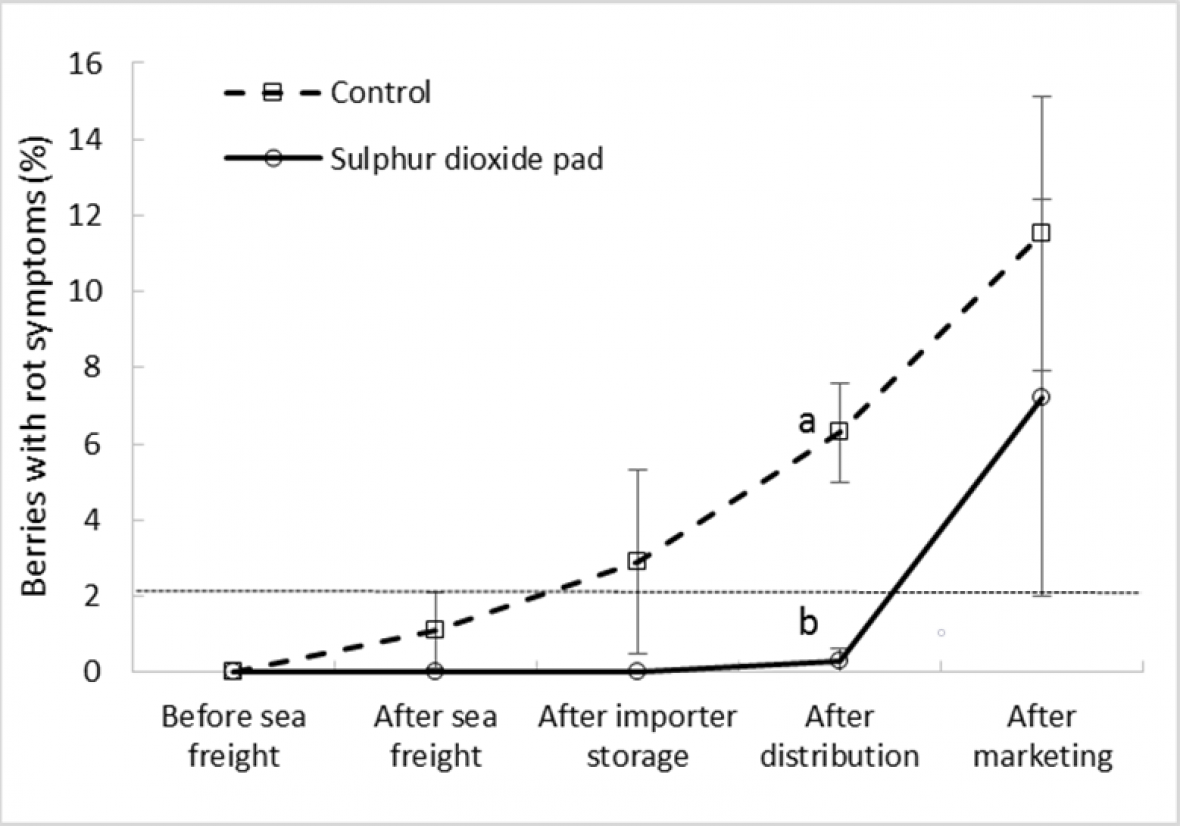
Figure 5b. Effect of SO2 treatment and export stage on bunch rot incidence in ‘Sweet Sapphire’ grapes; error bars show ± standard error of the mean from five carton replicates per treatment; different letters within a cultivar and export stage indicate a significant difference between treatments at P < 0.001; dotted horizontal line represents bunch ‘limit of marketability’ at 2 % incidence (i.e., approximately 10 berries with rots per bunch).
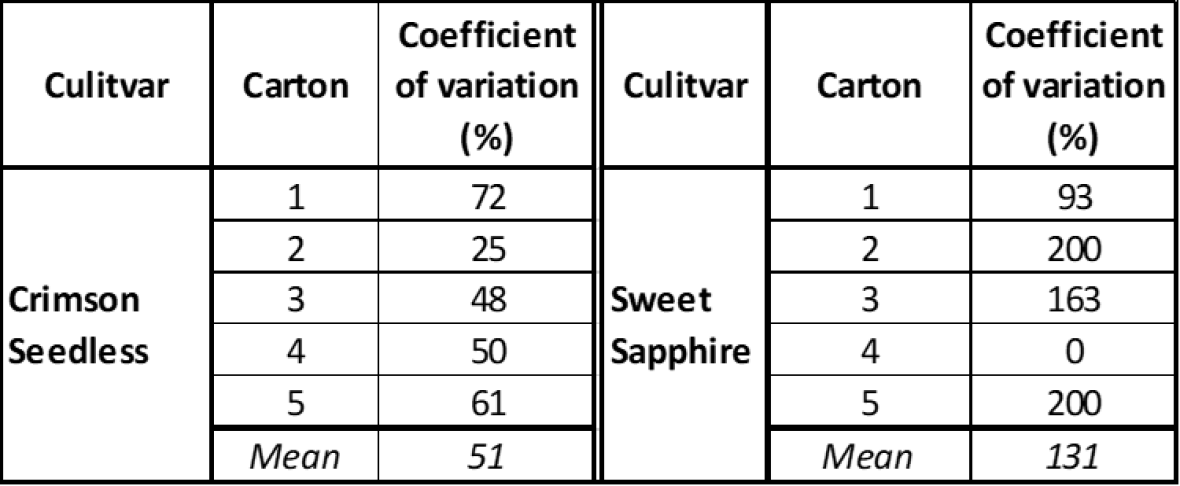
Table 1. Variability in rot incidence among cartons of ‘Crimson Seedless’ and ‘Sweet Sapphire’ after incubation at 18 °C for 6 days prior to sea freight simulation; coefficient of variation calculated from four bunches sampled and incubated from each carton.
‘Thompson Seedless’
Susceptibility to fungal rots during export simulation experiments in ‘Crimson Seedless’ and ‘Sweet Sapphire’ can be indirectly compared to cool storage and marketing experiments conducted on ‘Thompson Seedless’ stored for up to eight weeks at 0 °C (Fig. 6). ‘Thompson Seedless’ bunches were assessed fortnightly and then placed at 18 °C for three days to simulate a marketing period. Use of SO2 pads was again effective in reducing rot development, mainly Botrytis cinerea, in comparison to untreated bunches, but ‘Thompson Seedless’ appear to be more susceptible to rot development than either ‘Crimson Seedless’ or ‘Sweet Sapphire’ when rot incidence is compared after equivalent cool storage durations and marketing periods.
With extended cool storage, SO2 treatment appears to be more effective in supressing rot expression on berries in ‘Crimson Seedless’ and ‘Sweet Sapphire’ compared to ‘Thompson Seedless’. Differences in cultivar susceptibility is even clearer when comparing untreated fruit, with rot incidence of more than 25 % after six weeks of storage at 0 °C in untreated ‘Thompson Seedless’, which was double the incidence observed among the other cultivars after an equivalent storage duration incorporating sea freight and distribution stages.
The effect of higher storage temperatures on rot development in untreated ‘Thompson Seedless’ is demonstrated in Figure 7 where fruit were stored at 4 °C for up to eight weeks. Beyond two weeks of cool storage, an increase in cool storage temperature from 0 to 4 °C resulted in an increase in rot incidence among untreated fruit of 10 to 20 %, both directly out of cool storage and after marketing. Interestingly, SO2 treatment appeared to largely mitigate the impact of higher storage temperature on rot incidence for most of the cool storage duration, demonstrating the importance of this disease control measure in controlling postharvest rot development under variable temperature management during export.
Similar to both ‘Crimson Seedless’ and ‘Sweet Sapphire’, SO2 treatment of ‘Thompson Seedless’ appeared to have minimal effect on reducing rachis browning severity compared to no treatment during cool storage and marketing (not shown), with bleaching of rachides commonly observed among treated bunches in this cultivar after extended cool storage.
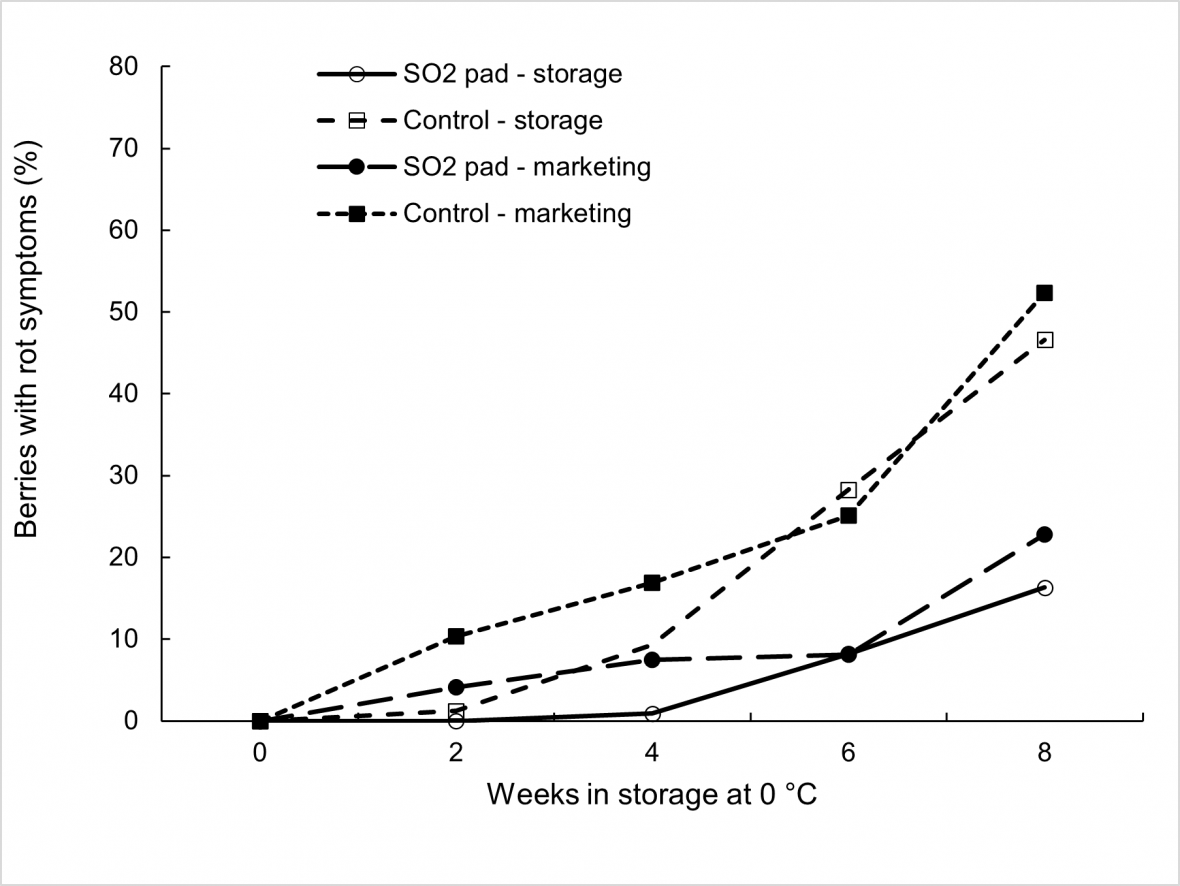
Figure 6. Effect of SO2 treatment, storage duration at 0 °C, and marketing for 3 days at 18 °C on bunch rot incidence in ‘Thompson Seedless’ grapes with each mean based on four replicate cartons.
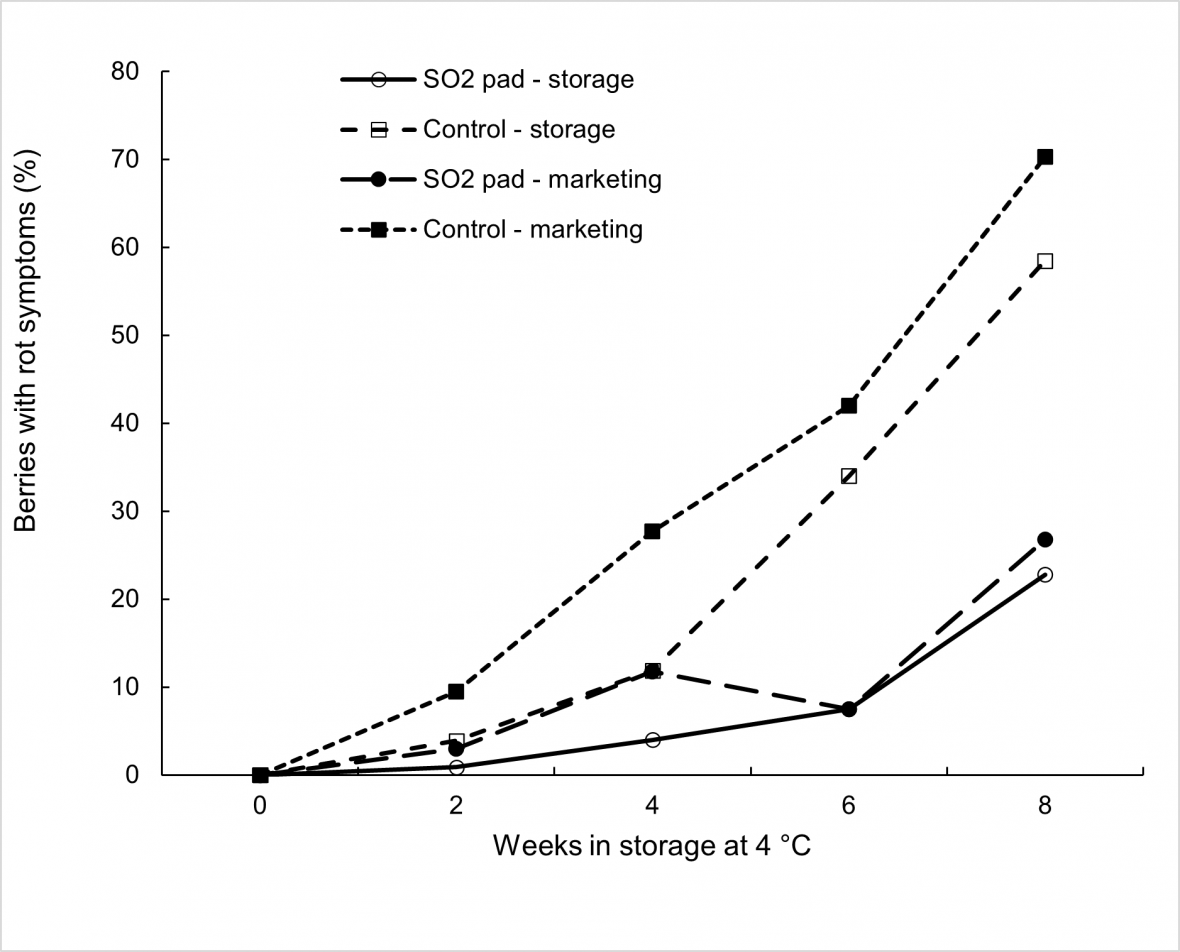
Figure 7. Effect of SO2 treatment, storage duration at 4 °C, and marketing for 3 days at 18 °C on bunch rot incidence in ‘Thompson Seedless’ grapes with each mean based on four replicate cartons.
Conclusions
- Table grape cultivars are susceptible to various fungal rots that can infiltrate developing berries in the vineyard (e.g., latent infection) or penetrate damaged berries during postharvest storage and handling (as berry skin weakens) or due to berry damage caused at harvest or during packing.
- Susceptibility to postharvest rot development is cultivar-dependent, with white cultivars tending to be more susceptible than red or blue cultivars, given similar vineyard management factors and harvest maturity.
- Appropriate use of SO2 treatment of all cultivars substantially reduces the potential for rot development during extended cool storage or along export cool chains.
- Within a cultivar, high variability in susceptibility to fungal rot development is likely within and between cartons, as demonstrated using incubation techniques at harvest, and likely resulting from differences in bunch position on the vine, differences in inoculum load at harvest and damage to berries during harvest and packing.
- SO2 treatment appears to have minimal impact on postharvest rachis browning severity in the studied cultivars, with rachide morphology (i.e.,lateral and pedicel thickness), postharvest conditions and cultivar likely to largely influence the rate of rachis browning.
- Within a cultivar, vineyard factors including bunch disease latent infection and inoculum load at harvest greatly influence postharvest rot development even when using optimum storage and SO2 treatment practices.
Acknowledgement
John Lopresti, Janine Jeager, Kristen Pitt & Glenn Hale
Literature cited
Crisosto, C., J. Smilanick, N. Dokoozlian and A.D. Luvisi (1994). Maintaining table grape post-harvest quality for long distant markets. International Symposium on Table Grape Production, American Sociaety for Enology and Viticulture, Anaheim, California, 195-199.
Lichter A., Y. Zutahy, T. Kaplunov and S. Lurie (2008). Evaluation of table grape storage in boxes with sulphur-dioxide releasing pads with either internal plastic liner or external wrap. Horttechnology, 18(2):206-214.
Project Acknowledgement
The Serviced Supply Chains project is funded by the Hort Frontiers Asian Markets Fund, part of the Hort Frontiers strategic partnership initiative developed by Hort Innovation with co-investment from: Department of Agriculture and Fisheries, Queensland; Department of Jobs, Precincts and Regions, Victoria; Manbulloo (mangoes); Montague (Summerfruit); Glen Grove (citrus); and the Australian Government plus in-kind support from The University of Queensland and the Chinese Academy of Sciences.